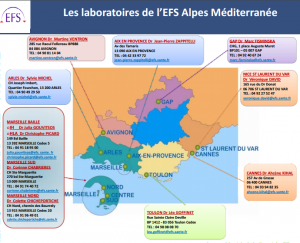
Dr Jean-Pierre Zappitelli is a pharmacologist and biologist, who works as the senior manager of the Aix-en-Provence laboratory of France’s national blood transfusion service, the Établissement Français de Sang (EFS). He discusses his work within the context of managing the cold chain for blood products
How does the EFS laboratory in Aix-en-Provence fit in with the rest of the EFS and how would you describe your work?
The EFS is France’s sole operator of blood transfusions and its work covers the processing of donations of blood, plasma and platelets. The EFS is France’s safety guarantor of the blood transfusion chain, from donor to recipient. It supports the care of over 1 million patients per annum across France and its overseas territories via 132 fixed and some 40,000 mobile donation sites.
 The EFS laboratory in Aix-en-Provence is a reception and distribution point of blood products that have been tested post-donation for pathogens and are ready to be used. In 2015, it processed 20,148 units of blood products (17,529 units of red blood cells; 1,148 units of platelets and 1,471 units of plasma) for the care of 3,759 patients.
The EFS laboratory in Aix-en-Provence is a reception and distribution point of blood products that have been tested post-donation for pathogens and are ready to be used. In 2015, it processed 20,148 units of blood products (17,529 units of red blood cells; 1,148 units of platelets and 1,471 units of plasma) for the care of 3,759 patients.
I’m the Aix-en-Provence laboratory’s senior manager responsible for delivery and distribution of blood products. By “delivery”, we mean blood products for specific named patients, while by “distribution” we mean for unnamed patients, for instance for hospital stock top-ups.
My work involves ensuring firstly that all of the products we receive are safe to use; secondly that while these products are in the laboratory’s care, they are stored correctly; and lastly, that they are correctly labelled and packed when they leave the laboratory.
How does the Aix-en-Provence laboratory manage the cold chain for the blood products in its care?
This is achieved by maintaining and monitoring the temperature in various areas in the laboratory.
Firstly, the ambient temperature in the laboratory itself is maintained at 25°C. We do this because this is the recommended temperature for the products that are used to determine blood type. If for some reason, this temperature level of 25°C is breached, an alarm sounds and dealing with this issue becomes the primary task that is attended to.
 Secondly, all units of blood products – platelets, red blood cells and plasma – are all kept in their own designated refrigerators. These all have a temperature indicator on their door, as well as an independent temperature monitor that is linked to an online map of all of the refrigerators in the laboratory, that is updated in real time with a full suite of data analytics.
Secondly, all units of blood products – platelets, red blood cells and plasma – are all kept in their own designated refrigerators. These all have a temperature indicator on their door, as well as an independent temperature monitor that is linked to an online map of all of the refrigerators in the laboratory, that is updated in real time with a full suite of data analytics.
The laboratory’s refrigerators have a safety feature whereby whenever one is opened and then closed, a magnet is activated and it cannot be opened for one minute in order to restore the correct ambient temperature within it.
Thirdly, all units of blood products come to the laboratory from regional stock in their own type of container with a “thermo-button” temperature data logger that has monitored the ambient temperature within every container we receive. These temperature data logger are checked using a sensor linked to a PC to ensure that the cold chain wasn’t broken prior to reception. (Some other laboratories in the region use RFID data loggers and for these, the crates don’t need to be opened for data monitoring downloads.)
 Also, all the bags of products in a group of bags are attached together with a self-locking cable tie that can only be removed by cutting it with pliers.
Also, all the bags of products in a group of bags are attached together with a self-locking cable tie that can only be removed by cutting it with pliers.
For blood products that leave our laboratory or may be returned, we group these units of blood products with both a self-locking cable tie and a thermo-button temperature data logger.
Lastly, every group of blood product bags is labelled with a tag indicating source, destination, required temperature and a security notice around handling. Also, every bag of blood product has its own barcode allowing for full traceability.
The laboratory also needs to consider how deliveries of bags of blood products are managed and for this we use two systems: either refrigerated vehicles or isothermal padding.
For bags of red blood cells and platelets, plastic crates are used for transportation in refrigerated vehicles. The crates contain the appropriate type of ice-pack to ensure optimal temperature levels within the crates. Also, these let in the cooled refrigerated air within the vehicle to further ensure that the correct temperature is maintained within them.
For deliveries of bags of frozen plasma, air-tight isothermal crates with extra isothermal padding and its appropriate type of ice-pack. Also, all units of frozen plasma are protected with bubble wrap to avoid any breakages during transportation.
Lastly, all crates are labelled with information regarding source, destination, required temperature and a security notice around handling. Naturally, only authorised personnel can open a crate and this too is indicated on every crate.
How does the laboratory manage suspect items?
The main rule we work by is to eliminate all doubt around decision making about a product. Either it is objectively safe to use or it isn’t, and both of these scenarios are backed up by data, or a lack thereof.
 Until we can determine that a suspect unit of product is safe to use, it’s placed in a quarantine refrigerator until its safety has been 100% confirmed. If this can be done, it then is moved to the appropriate refrigerator. If this cannot be done, it’s disposed of by means of incineration.
Until we can determine that a suspect unit of product is safe to use, it’s placed in a quarantine refrigerator until its safety has been 100% confirmed. If this can be done, it then is moved to the appropriate refrigerator. If this cannot be done, it’s disposed of by means of incineration.
Also, any such suspect product, which goes on to be disposed of, is fully documented to ensure full traceability.
In the lab, we make sure that the technicians who process the products we process are never in any doubt about what course of action they need to take.
Our safety systems are built to enable any of our processes to stop at any point. There’s simply no room for error. Patients’ lives are at stake.
What are the risks if the cold chain is broken (plus or minus)?
The risks associated with managing the cold chain for blood products underpins all our work.
If the cold chain for a bag of red blood cells is broken, say during transport, and its core temperature rises above 10°C, the risk is that the patient receives a transfusion that is infected by potentially harmful bacteria. And even with minute amounts of bacteria, at temperatures above 10°C bacterial reproduction can be considerable. For a healthy person, this needn’t necessarily be a problem, but for an already weakened sick patient, it can be extremely dangerous. And for temperatures below 2°C, the risk here is that ice crystals will form, thus impairing its oxygenation property.
Similarly, if platelets are stored or transported at temperatures below the specified limit, there is also an inefficiency risk. So if for example these are stored or transported at a temperature of 12°C instead of 24°C, the purpose of the transfusion (to replace platelets further to some chemotherapy therapy) will be adversely affected, which again poses a danger to the patient. And just like red blood cells stored or transported at a temperature above 24°C, the risk is bacterial reproduction is very high.
 For plasma, the risk when the cold chain is breached is that the product will be altered very rapidly. Plasma is an extremely fragile product that must be used within six hours of being unfrozen.
For plasma, the risk when the cold chain is breached is that the product will be altered very rapidly. Plasma is an extremely fragile product that must be used within six hours of being unfrozen.
The final risk around managing the cold chain for blood products is around the labelling of each item. This is why we use various identification tools, including unique barcodes on every bag of blood product. During transport for instance, correct labelling ensures the timely and correct delivery of blood products.
When this doesn’t happen, stocks of blood products could be either delivered to the wrong destination, or arrive late, leading to patients not benefiting from them when they need them or a hospital’s stocks running low, or worse running out, which again puts patients at risk. Furthermore, incorrect labelling inevitably leads to unnecessary wastage to eliminate any chance of putting a patient at risk with a potentially unsound product.
Managing these risks is why for every unit of blood product, there has to be absolute certainty regarding where it’s come from and what its destination is. There’s no room for any grey areas in our work. We only work with 100% certainty around the safety protocols for the blood products we process.



 Helap
Helap Supplied in 5 different sizes VaccinePorter® Carriers can accommodate up to 8 litres of product. Pick out your preferred size by requesting your hard copy of our VaccinePorter brochure, simply by emailing
Supplied in 5 different sizes VaccinePorter® Carriers can accommodate up to 8 litres of product. Pick out your preferred size by requesting your hard copy of our VaccinePorter brochure, simply by emailing  The EFS laboratory in Aix-en-Provence is a reception and distribution point of blood products that have been tested post-donation for pathogens and are ready to be used. In 2015, it processed 20,148 units of blood products (17,529 units of red blood cells; 1,148 units of platelets and 1,471 units of plasma) for the care of 3,759 patients.
The EFS laboratory in Aix-en-Provence is a reception and distribution point of blood products that have been tested post-donation for pathogens and are ready to be used. In 2015, it processed 20,148 units of blood products (17,529 units of red blood cells; 1,148 units of platelets and 1,471 units of plasma) for the care of 3,759 patients. Secondly, all units of blood products – platelets, red blood cells and plasma – are all kept in their own designated refrigerators. These all have a temperature indicator on their door, as well as an independent temperature monitor that is linked to an online map of all of the refrigerators in the laboratory, that is updated in real time with a full suite of data analytics.
Secondly, all units of blood products – platelets, red blood cells and plasma – are all kept in their own designated refrigerators. These all have a temperature indicator on their door, as well as an independent temperature monitor that is linked to an online map of all of the refrigerators in the laboratory, that is updated in real time with a full suite of data analytics. Also, all the bags of products in a group of bags are attached together with a self-locking cable tie that can only be removed by cutting it with pliers.
Also, all the bags of products in a group of bags are attached together with a self-locking cable tie that can only be removed by cutting it with pliers. Until we can determine that a suspect unit of product is safe to use, it’s placed in a quarantine refrigerator until its safety has been 100% confirmed. If this can be done, it then is moved to the appropriate refrigerator. If this cannot be done, it’s disposed of by means of incineration.
Until we can determine that a suspect unit of product is safe to use, it’s placed in a quarantine refrigerator until its safety has been 100% confirmed. If this can be done, it then is moved to the appropriate refrigerator. If this cannot be done, it’s disposed of by means of incineration. For plasma, the risk when the cold chain is breached is that the product will be altered very rapidly. Plasma is an extremely fragile product that must be used within six hours of being unfrozen.
For plasma, the risk when the cold chain is breached is that the product will be altered very rapidly. Plasma is an extremely fragile product that must be used within six hours of being unfrozen.